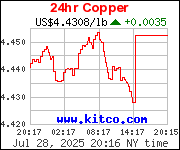
In previous weeks I have been touting the advantages of investing in copper, by accumulating mostly copper-bearing cents. The Lincoln cent, to be precise. And specifically those minted before 1982. Prior to 1982, the cent was comprised of 95% copper, 5% zinc. The purist will point out that - for years - a tiny bit of tin was thrown into the mix as well, but that trace amount was so small as to be considered inconsequential.
Pennies minted prior to 1982 are thus considered "copper." In fact, they are bronze, but let's not quibble over semantics. In 1982, the United States Mint, fearing that the price of commodities was about to spike, preemptively discontinued the copper cent. They estimated that it would prove more economical - providing annual cost reductions of about $25,000,000 - to produce cents from a different alloy. Subsequently, pennies fabricated as of late October, 1982 consist mostly of zinc. Their precise composition is 97.5% zinc, 2.5% copper.
At present, these "zinc" cents serve the public best with their original intent. Spend them. That's what they were made for, that's what they're good for. After all, if the penny were to vanish overnight, in short order all sorts of repercussions would ripple throughout commerce. Prices would have to be rounded, either up or down. Merchants are out to make money. So prices would likely creep up a few more percent, assisted by such a disappearance from circulation.
But this leaves a gap in our thinking. Pre-1982 "good" cents, post 1982 "bad" pennies. Think Gresham's Law here. What about 1982 itself? We learn from the cents census - my source being Coins Magazine - that in 1982 the Philadelphia Mint produced 10,712,525,000 cents. That's a lot of pennies, by any count. But it doesn't end there. The Denver Mint added another 6,012,979,368 coins to the fray. Lastly, the San Francisco Mint bolstered that count by another 3,857,479 cents.
That number, represents "proof" cents; coins produced for collectors. A proof coin will generally display a mirror-like field. The raised devices will be frosted. Such coins are produced using a high-quality minting process which involves a special strike. It would be disingenuous to add this small total to the final tally, as these cents were never intended for general circulation. For every 1982-S cent that you find, it means that someone had to intentionally break apart a proof set.
Are these 1982 cents then - roughly seventeen billion - worth the bother of saving? More cents were minted in 1982 than ever before, or after. So it's kind of hard to ignore them. But it does require a bit of effort to determine whether a cent is copper. Is it worth the trouble? Would it be better just to disregard the 1982's entirely, even though 75% are copper? Maybe we should only pull 1981's and earlier from circulation? What are the pros and cons of saving 1982 pennies? I mean, after all, what are they worth anyway?
Coinflation.com is an Internet site which provides an answer to this question. I find it to be a very useful reference source, as well as one filled with fascinating details. They post articles relevant to collectors and, daily, they recalculate the value of the metals intrinsic to all United States coins. The illegality of melting circulating American coins is not at issue here, Coinflation merely supplies these answers to satisfy the curiosity of the public.
From Coinflation, then, we learn the current "worth" of a 1982 penny. The melt price for scrap copper - on the day this was written - was $3.3397 per pound. Based on that price, the calculated worth of a 1982 penny is 2.21 times face value. Or is it 0.636? Well, I guess it all depends whether you're holding a predominately copper penny, or one made mostly of zinc.
Quite a difference, wouldn't you say? Copper pennies are worth three and a half times zinc? Well, then I would think that builds a case to sieve the copper 1982's from the zincs, if it wasn't too much extra effort. How do we go about that? This, I'm ashamed to admit, is another pun on my part as regards the title of this piece. We might need to make use of a jewelers scale.
Copper pennies weigh 3.11 grams. They are heavier than the zinc, which weigh only 2.5 grams. Unless you wield the sensitivity of Liberace - and could discern the weight differential of two butterflies perched upon your fingertips - you won't be able to distinguish this 0.61 gram differential by comparison of their heft. You'll need an accurate means of weighing them. This is where a gram scale comes in. They're really not that expensive.
Mine cost $21.99 with free shipping. I found it on ebay. I'ts a Mini Digital 600 gram scale. When set in gram mode, the DigiWeigh DW 600BS can determine weight differentials of 0.1 grams. This is sufficient to accurately determine whether a cent is copper or zinc. I'm confident that similar products from reputable sellers (check their feedback) are available. I'm not endorsing any particular brand. I mention this one only because it's the one I own.
Having access to a digital scale can help you avert alternatives. You could construct a simple balance yourself. I have to credit HoardCopperByTheTon - a realcent forum administrator revered for his tongue-in-cheek posts - who weighed in with this suggestion. Place a popsicle stick, centered, across a pencil. Then place a known copper penny on one end, a 1982 on the other. Like a teeter-totter, the copper will win if zinc is on the other side. If, after a moment of quivering, the stick settles and remains horizontal, then you've got two coppers.
I find this to be too much trouble. The one time I tried it I felt like a klutz. But for some it might offer a cheap solution. You could also drop the suspects onto a hard surface. The copper cents will produce a distinct resonant ring upon impact, as opposed to the dull thud of zinc. A third method to differentiate relies on sharp scrutiny. There's a difference in the numerals and lettering. You say you'd like more details?
An instructive primer may be viewed at http://www.thecentproject.com/sort1982.php.
A thread found on Collector's Universe adds some humor to the debate. It's a long url - you'll have to paste the following two segments into one continuous link to gain access - but it's worth it. http://forums.collectors.com/messageview.cfm?catid=26&threadid=224143&highlight_key=y&keyword1=1982%20cent
Now that we have dispensed with determining whether to save 1982 pennies, let's discuss a related topic. From Wikipedia we learn that "in the United States at the end of 2001, 10% of the population owned 71% of the wealth, and the top 1% controlled 38%. On the other hand, the bottom 40% owned less than 1% of the nation's wealth." End quote; enter segue. Why save cents at all?
Copper, like silver and gold, will invariably rise in value, though not to the same heights. As inflation continues, this is virtually assured. It wouldn't surprise me if hyperinflation was someday to be our fate. If that were the case, your copper penny piles would serve twin purpose. They would be useful in barter, as opposed to worthless paper fiat currency. And their value could increase by multiples.
But, saving copper cents? Wouldn't you have to do it on a huge scale to make it worthwhile? Isn't it all rather, ahem, penny ante? The large, well-heeled investor, would probably scoff at this concept. But not all individuals have the capital to gamble on futures, or speculate on mining equities. Some can't even afford a small amount of silver or gold bullion. Should that exclude them from participation in the projected rise in the value of real money commodities? I think not.
Gathering and keeping copper cents is an ideal means of, literally saving your money. Saving your purchasing power by holding onto a tangible metallic form of money. Thus, accumulating this type of copper bullion is ideal for the small investor, even those with miniscule income. As long as they have two cents to rub together they can join in. If you can't afford silver and gold, by all means, save copper. It's still investing, just on a different scale.
Buy Silver. Buy Gold. Save Copper. Start Now.
Friday, November 20, 2009
Subscribe to:
Post Comments (Atom)


























































































































































No comments:
Post a Comment